Keith Alcorn July 24, 2019
Treatment with dolutegravir resulted in weight gain for participants in two large clinical trials in sub-Saharan Africa and was especially pronounced when dolutegravir was combined with the new formulation of tenofovir (tenofovir alafenamide fumarate, TAF), Dr Michelle Moorhouse of Wits Reproductive Health Institute, Johannesburg, reported on Monday at the 10th International AIDS Society Conference on HIV Science (IAS 2019) in Mexico City.
In both studies, women gained more weight than men.
Dolutegravir is a highly potent HIV integrase inhibitor that is recommended for first-line antiretroviral therapy in all major treatment regimens. At the conference, the World Health Organization revealed updated guidelines recommending the use of dolutegravir in the treatment of all adults.
Since dolutegravir (Tivicay) received marketing approval in the United States and the European Union in 2013, several side effects have been observed in cohort studies.
Central nervous system side effects, such as insomnia, anxiety, depression and dizziness, have been reported at higher levels than in the studies that led to marketing approval.
Neural tube defects in infants exposed to dolutegravir at conception or in the first three months of pregnancy were observed more frequently compared with infants exposed to other regimens in an observational study in Botswana, but this safety signal has not been demonstrated in cohort studies in other countries.
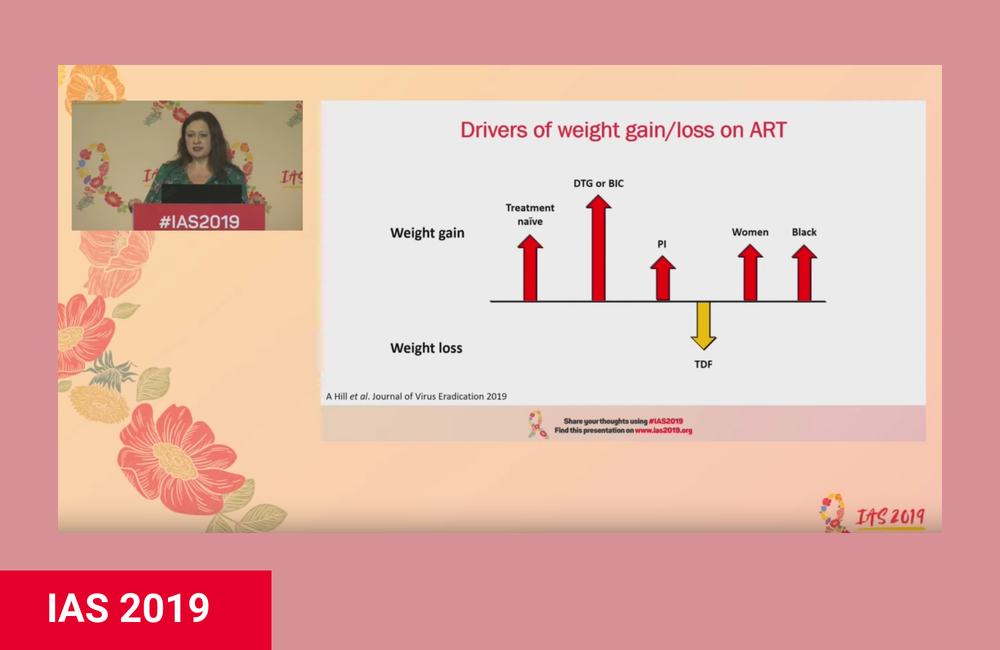
Weight gain associated with the integrase inhibitor dolutegravir has been observed in several large cohort studies, but evidence from randomized clinical trials has been less clear.
Weight gain after starting antiretroviral treatment is common, but substantial weight gain can increase the risk of adverse birth outcomes, diabetes, cardiovascular disease and some cancers. Clinical obesity, defined as a body mass index (BMI) greater than 30, has been shown to reduce life expectancy by four years.
Dr Andrew Hill of the University of Liverpool and other researchers looked at weight gain after starting dolutegravir treatment compared with efavirenz treatment in two clinical trials in sub-Saharan Africa.
NAMSAL Study
The NAMSAL trial randomly assigned 613 adults in Cameroon to either the older formulation of tenofovir (tenofovir disoproxil fumarate, TDF), lamivudine, and dolutegravir, or tenofovir, lamivudine, and efavirenz. Participants' body weight was measured at baseline and at week 48.
The study population was approximately two-thirds women with a median BMI of 23 (IQR 21-26). Thirty percent of participants had a very high baseline viral load (>500,000 copies/ml).
Después de 48 semanas, los participantes en el brazo de dolutegravir del estudio habían ganado una mediana de 5 kg en comparación con un aumento de 3 kg en el brazo de efavirenz (p <0,001). El IMC aumentó en +1.7 en el grupo de dolutegravir y en +1.2 en el grupo de efavirenz (p <0.001). No hubo diferencia en las proporciones que ganaron suficiente peso para ser clasificadas como sobrepeso (IMC> 25), pero una proporción significativamente mayor del grupo de dolutegravir ganó suficiente peso para ser clasificada como obesa (IMC> 30) (12 vs 5%) ( p <0,01).
El aumento de peso de más del 10% de la masa corporal fue significativamente más probable en las mujeres que tomaron dolutegravir (44 vs 34%, p <0,05) en comparación con efavirenz, pero no hubo diferencias significativas en el aumento de peso según el régimen en los hombres (26 vs 19%).
Por otro lado, la aparición de obesidad en el tratamiento difería según el régimen solo en los hombres, y el dolutegravir se asoció una vez más con un mayor aumento de peso (14 vs 2%, p <0,01).
ADVANCE Study
The ADVANCE study randomly assigned 1,053 adults and adolescents in South Africa to tenofovir disoproxil fumarate (TDF), emtricitabine, and dolutegravir; tenofovir alafenamide fumarate (TAF), emtricitabine, and dolutegravir; or TDF, emtricitabine, and efavirenz. Participants' body weight was measured at baseline and every 12 weeks. A substudy used DEXA scans to measure limb fat at baseline, week 48, and week 96.
The majority of the study population was female (57–61% per study arm) and women had a higher mean body weight and BMI than men (male BMI 21.7, female BMI 25.6–26.1). Approximately 35% of participants were clinically overweight or clinically obese.
After 48 and 96 weeks, weight gain was greatest in the TAF/emtricitabine/dolutegravir arm (+6 kg, +8 kg). Weight gain was least in the TDF/emtricitabine/dolutegravir arm (+3 kg, +5 kg) and lowest in the TDF/emtricitabine/efavirenz arm (+1 kg, +2 kg).
De manera similar, la proporción de personas con sobrepeso clínico u obesidad clínica fue mayor en el brazo de TAF / emtricitabina / dolutegravir. A las 96 semanas, el 19% en el brazo de TAF se había vuelto clínicamente obeso, en comparación con el 8% en el brazo de TDF / emtricitabina / doltegravir y el 4% en el brazo de efavirenz (p <0,01).
In the ADVANCE study, there was a significant difference in weight gain by gender. Men and women randomized to dolutegravir gained weight through week 48, but while men's weight stabilized after week 48, women continued to gain weight.
Clinical obesity occurred more frequently in women during the follow-up period, especially in women randomized to tenofovir alafenamide. In the TAF group, 20% of women became clinically obese at week 48 and 23% at week 96, compared with 8% of men at weeks 48 and 96.
Body composition analysis using DEXA showed that just over half of the weight gained by men in the dolutegravir arms was fat and this was evenly distributed between the trunk and limbs. In women, DEXA scans showed that around two-thirds of the weight gained in the dolutegravir arms was fat, again evenly distributed between the trunk and limbs.
Multivariate analysis controlling for demographic factors, baseline BMI, viral load, and CD4 count, concomitant medications, and history of diabetes, hypertension, and elevated cholesterol, showed that treatment-associated obesity was associated with TAF/emtricitabine/dolutegravir treatment, baseline viral load or CD4 count, and baseline BMI. A weight gain of 10% or more was associated with TAF/emtricitabine/dolutegravir treatment, baseline viral load or CD4 count, female sex, and baseline weight.
However, when 68 participants in the ADVANCE study were asked about their perceptions of weight gain, few complained. Eight of the 51 women expressed unhappiness, although only four had gained more than 10% of their body weight. In some cases, study participants saw their weight gain as a sign of a return to health, even when they were not underweight, or experienced weight loss before starting treatment. For many participants interviewed, the main consequence of weight gain was the need to buy new clothes, as their pretreatment clothes no longer fit.
Longer-term follow-up is needed to assess the consequences of weight gain in these study populations. Follow-up of ADVANCE study participants will continue for two more years, but analysis of other trial populations is needed to look at weight gain in different settings and populations, Moorhouse said.
References
Hill A et al. Progressive weight gain and clinical obesity for TAF/FTC/DTG and TDF/FTC/DTG versus TDF/FTC/EFV: ADVANCE and NAMSAL trials. 10th IAS Conference on HIV Science, Mexico City, abstract MOAX0102LB, 2019.
At: http://www.aidsmap.com/news/jul-2019/dolutegravir-leads-weight-gain-two-african-studies